What is Hydrogen Decrepitation
Hydrogen is a very small and very reactive atom (the smallest atom- comprised of just one proton and one electron) that easily penetrates the grain boundaries of many metals. In most situations, metallurgists try to prevent hydrogen from entering the metal.
Hydrogen embrittles metals by entering the grain boundaries and creating pressure at the weakest point. This causes micro-cracks that begin to propagate through the grain structure.
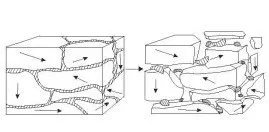
Then why would you want to introduce hydrogen
into a metal? Because we can use the same property to achieve our goal of
reducing the grain size. If we deliberately introduce a large amount of
hydrogen into the metal, it actually falls apart –decrepitates- because the
hydrogen is so effective at breaking the metal down into very small pieces.


