What happens when you approach the blood with a magnet?
Iron is ferromagnetic and can be
attracted by magnets, so many people will assume that iron-containing blood
must also be magnetic. In the movie "X-Men", Magneto once controlled
his actions by manipulating the iron in the guard's blood. But is blood really
attracted by the magnetic field?
In
November 1845, Faraday conducted an experiment to test whether the blood is
magnetic. Faraday wrote in his diary: "Blood has no magnetism, I am
shocked... Considering that iron is magnetism in almost all states, this is
even more surprising.

Faraday actually found only half of the
truth. 91 years later, another big man, American chemist Linus Pauling, who won
the Nobel Prize twice, and his colleagues discovered that the magnetic
properties of arterial blood and venous blood are also different. Compared with
venous blood, arterial blood Blood is more easily repelled by magnets. In
physics, the property that is attracted by a magnet is called paramagnetism,
and the property that is repelled by a magnet is called diamagnetism. In fact,
the word "diamagnetic" was also invented by Faraday when he was
playing with a drop of blood in 1845. Faraday also discovered that all
substances have varying degrees of diamagnetism: water, most organic compounds,
metals, and mercury are all repelled by
magnets.
The diamagnetism of the graphite in the
pencil allows them to levitate on the magnet. Among all substances with
diamagnetism, superconductors have super diamagnetism.
To determine whether a substance is
diamagnetic or paramagnetic is simple: just look at whether its molecules have
unpaired electrons. Unpaired electrons mean single electrons, that is electrons that do not form an electron pair in the molecular orbital. As we all
know, being single is agitated, and single electrons are the same, prone to
chemical reactions. For example, artemisinin uses free radicals produced by
single electrons to kill malaria parasites.

In addition, single electrons are also
prone to lose their stamina and are attracted by the magnetic field, making the
molecules paramagnetic. In other words, if all electrons in a molecule are in
pairs, then it has diamagnetism; conversely, if there are unpaired electrons in
a molecule, then it has paramagnetism. Mercury and gold are metals, but they
are diamagnetic because they have no unpaired electrons. We usually do not
observe gold and mercury being repelled by the magnet, mainly because its
diamagnetism is relatively weak.
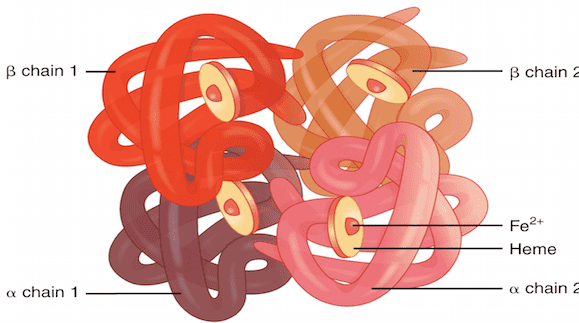
So what is the condition of human blood?
Hemoglobin has 4 iron-containing subunits. Oxygen O can be attached to each
subunit. Subunits with rings are called oxyhemoglobin, and those without rings
are called deoxyhemoglobin.
Pauling and his colleagues discovered that oxyhemoglobin is diamagnetic, but deoxyhemoglobin is paramagnetic because iron atoms not covered by oxygen contain unpaired electrons. Therefore, the paramagnetism of venous blood is stronger than that of arterial blood.
The reason why you are not attractive: The electrons in the body are all in pairs.


